A Contribution
to the Geographical
Interpretation
of Biological Change
Charles H. Smith
[[Author's Note: Originally published in Acta Biotheoretica
(Volume 35: 229-278, 1986), and reprinted here verbatim. Original pagination
noted within double brackets.]]
[[p. 229]] KEY
WORDS: geography, evolutionary theory, adaptation, biogeography, individualistic
hypothesis, resource cycling.
ABSTRACT: "Geography" has traditionally been assigned the role of handmaiden in evolutionary studies. In this work a different understanding of the relationship between biological change and locational setting is developed: evolution as a dynamic form of spatial interaction. In the causal model presented, adaptive change is portrayed as a negative feedback response contributing to a general spatial-temporal process of resource cycle tightening involving exchanges between the two fundamental structural sectors ("abiotic" and "biotic") of the earth's surface system. As such, it is rejected as "evolution" per se. This position makes it possible to circumvent the "adaptation yields adaptation" circularity, and to view locational circumstances as being evolutionarily causal, yet not deterministic with respect to population-level change. A parallel interpretation of the relation between range and range change and evolution is also implicit; the individualistic hypothesis is thereby superceded by a model of community evolution allowing for individualistic rates of population (adaptive and) range change, but operating on the principle that populations should tend to change range in common directions (as a response to spatially-varying degrees of efficiency of turnover of resources vital to biotic sector function). This in turn leads to the possibility of normative biogeographic modelling. Comment is also made on the relationship of the present understanding to disequilibrium and dynamic equilibrium interpretations of evolutionary change, and to human cultural evolution.
[[p. 230]] 1. INTRODUCTION
One of the most interesting aspects of the study of evolution can be summed up in the question "What is evolving?" For over a century our views on this subject have derived largely from the theory of natural selection, which focuses on the characteristics of organisms and populations. And yet it seems clear that evolution may be understood to operate at other scales as well; for example, at the world ecosystem, "macroevolutionary" [108], and community [125] levels. Moreover, even the history of ideas on population-level change itself has undoubtedly been strongly influenced by prevailing opinions on the relation of that change to extra-population levels of organization. Within such discussion the role of "geography" in evolution, in particular, has been much debated; as a result, that role has come to be interpreted in quite different ways by various workers. For paleobiologists [e.g., Simpson: 102, 103] it is a changing stage setting through which passes a progression of phyletic divergence and extinction. For ecologists, on the other hand, it is the stage setting itself that is considered more worthy of recording; the roles of individual members of the cast are viewed as incidental in the chain of overall causal process.
Whatever our particular leanings on this matter, however, it seems that on the whole we have fallen into the habit of accepting the basic legitimacy of the "stage" concept. I question whether this relegation of geography to the status of a handmaiden is in the best interest of continuing refinement in evolutionary modelling. In this work an almost entirely deductive style of argument is used to build a model of physical-biological interaction that lends itself to various kinds of study of organismal change and distribution. Although the object of the overall discussion is to develop a more appropriate framework through which to interrelate spatial setting and evolution, our point of departure must be at the level of entirely aspatial considerations.
[[p. 231]] 2. THE BIOTIC/ABIOTIC SECTOR MODEL
We begin by focusing our attention on a simple model of the general flow of energy and mass through the earth's surface system. Figure 1 gives one means of portraying the relevant relationships; this framework is taken as given in all discussion to follow. As outlined in this diagram, energy and/or mass on the earth's surface are viewed as circulating through two delimitable sectors, the "biotic" and "abiotic", and across two interfaces (between the abiotic sector and the extra-planetary environment, and between the biotic sector and the abiotic sector). The term "biotic sector" is defined here simply as the world sum of that which is living organism. All of that on the earth's surface which is not living, metabolizing organism -- including organic
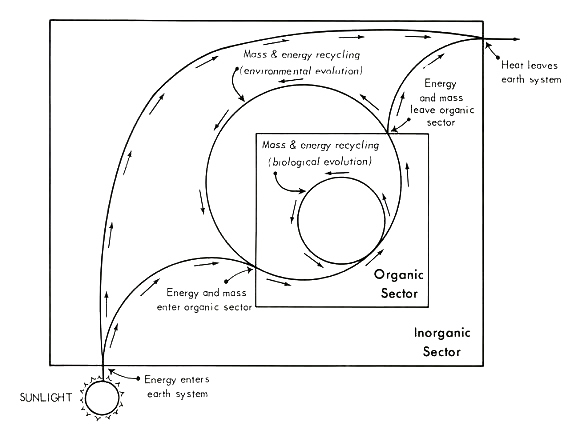
Figure 1. A general representation of mass and energy flow through the earth's surface system, with the latter envisioned as divided into two subsectors [after 104, p. 35]. See text for discussion.
[[p. 232]] wastes, carrion, not-yet-assimilated ingested foodstuffs, etc. -- is assigned to the "abiotic sector".
The structure depicted in Figure 1 is flexible enough to sustain both state-space and process interpretations. The state-space view conveys an ecological, or cross-sectional, interpretation in which the system is understood as: 1) open with respect to energy flow and closed with respect to material flow, and 2) operating under steady-state conditions. Analysis of state-space infrastructure proceeds under the assumption that there be no irreversible change in the input-output relations of the system over the period studied [8, 76, 77, 99]. Under such uniformitarian constraints, when subsystems of finite lifespan reach the end of their usual terms of existence, they are replaced by like entities (or, at the least, by entities realizing an equivalent function).
Cross-sectional studies usually explore the means through which living systems maintain equilibrium under ranges of conditions imposed on them by external forces. In such work, the internal differentiation of the system is ordinarily viewed as contributing to system "invariability" [116]. This perspective leads to a view of organismal function dominated by a "deviation-from-the-norm" kind of thinking; i.e., that the dynamics of particular biological subsystems can be stated in terms of ranges of input to, and output from, the unit [2, 21, 30, 32, 116]. To one degree or another, therefore, studies linking the biological state of organisms to the state of their immediate environment are implicitly amplifications on the theory of limiting factors [110].
When the investigator wishes to examine processes involving irreversible change, the cross-sectional approach depicted above proves too confining. In this kind of work, analysis of the way intrasystem feedback controls develop becomes necessary. As Carson [17, p. 76] remarks:
". . . A system may achieve equilibrium between form and process (assuming that the external variables which control the processes do not change) almost immediately in some cases; in other instances, the system may proceed so slowly towards equilibrium that an evolutionary approach is necessary to understand the nature of the system at any [[p. 233]] one point in time. In the situations where a system rapidly achieves equilibrium between form and process, an evolutionary model is unnecessary and a complete understanding of the nature of the system is furnished by a knowledge of the way in which the equilibrium pattern depends upon the external variables. An exception occurs when the outside variables themselves change through time in a systematic manner: although it is still possible to understand the nature of the system at any one point in time by reference to the current state of the controlling variables, a more complete explanation is afforded by setting the system in a historical framework."
In the above passage Carson introduces two ideas that are crucial to the present discussion: 1) that evolutionary (irreversible) change in a system may be linked to controls set by exogenous variables, and 2) that irrespective of such change, the current state of the system can always be related back to those same variables. Maruyama [76, 77] and Zadeh [128] have developed similar ideas. The notion that exogenous factors might direct organic evolution is not a new one, of course, but historically the tendency has been to dwell on the way these might deterministically exert influences on the development of individual populations [15, 22, 55, 66]. Given the usual association between the limiting factor notion and population-level influence, it is not difficult to understand why this has been so. It can be argued, however [104], that this way of thinking usually leads to little more than the identification of proximate causation between adaptive response and environment, a framework that works against efforts to unravel the causal basis of organic change. A different tactic will be employed here to develop a base for evolutionary interpretations of Figure 1. We shall begin with the idea that the biotic sector as a whole evolves in response to constraints set by the abiotic sector. The argument to follow will deliberately avoid citing particulars regarding what we normally consider the "characteristics" of biological evolution (i.e., the temporal unfolding of lineages and appearance of associated suites of adaptive innovations); the reason for maintaining this strategy should become apparent as the discussion develops.
[[p. 234]] 3. SYSTEM CONTROLS AND EXCHANGES AS VIEWED THROUGH THE TWO SECTOR MODEL
It is relatively easy to isolate the terms under which a system in steady-state with its environment operates: under steady-state conditions the amount of negentropy imported to the system must remain equal to the entropy produced by it [21, 25, 54, 85]. This constraint limits the changes possible within the system to uniformitarian kinds of adjustment; i.e., to the aforementioned maintenance process characterized by replacement of "worn-out" subsystems by subsystems of like structure and function (in the dynamic equilibrium steady-state case, by subsystems of like function alone).
Characterizing a system changing in an ordered fashion through time is a more complicated matter, as change must be explained in the face of the system's continuing ecological / thermodynamic equilibrium. As Huggett [54] points out, the very word "equilibrium" implies absence of change, yet at some level of organization every system is undergoing change. The earth as a whole, for example, operates under very nearly steady-state conditions with respect to its total energy throughput; nonetheless, its surface, at least, has undergone a continual process of evolution since it first came into being. Can we conclude from this historical fact that steady-state conditions have not actually been reached within the earth's surface system? If we do, the first effect of the resulting paradox is to leave us with a problem regarding terminology. Some geomorphologists [see discussion in references 18 and 54] have attempted to get around this difficulty by viewing systems whose input-output balance changes only very slowly with time as being in a state of dynamic equilibrium, but this solution seems to complicate the issue rather than clarify it. For now, let us assume that for each restricted time period and / or spatial setting, at least, something closely resembling dynamic equilibrium steady-state conditions does exist. This cannot, however, prevent us from emphasizing the nonequilibrium characteristics of the earth's surface when we focus on the changing [[p. 235]] nature of its component subsystems. At the end of this work, I will suggest a reinterpretation for this awkward situation.
The preceding characterization seems consistent with the notion that the earth's surface may be acting out a slow evolution toward largely non-evolutionary conditions. It is certainly not difficult to picture the historical trend of global events as a progression toward a steady-state material turnover regime; if this be an accurate representation, what can we infer about the nature of associated changes in the biotic sector? Moreover, what might be the implications for biotic sector dynamics when the steady-state is reached? I submit that we cannot attempt to satisfactorily answer this question without a refined understanding of the relation between "geography" and evolutionary process. In the interest of moving in that direction I shall attempt to provide a model of the (assumed) dis-equilibrium attending evolution that can be used as the basis for making statements about the way organisms change and are distributed in space. We need first give further attention to the general dynamic setting of the biotic sector.
Though the emphasis here is on the evolution of the biotic sector, it is apparent that the conditions underlying change in it and the abiotic sector are mutually causal [following 77]: both energy and material resources move through each and back and forth from one to the other. As a result, intra-sector processes in each may be viewed as exogenous variables with respect to their influence on the operation of the other. Nonetheless, the two differ in that the abiotic sector as defined earlier represents the only set of exogenous influences on biotic sector organization (whereas input to the abiotic sector originates in both the biotic sector and extra-system sources, especially the sun). This fact makes it easier to establish a simple causal model of biotic sector evolution. To maintain high levels of order in a living system, negentropy must be imported to it [8, 63, 77, 99]. It follows from initial definitions that all such import to the biotic sector must pass through the interfaces [[p. 236]] between the latter and the abiotic sector. Across these must move the resources that are necessary to the maintenance of biological activity; these have been "made available" to the biotic sector through the operation of return pathways that have synergistically evolved within the abiotic sector (e.g., biogeochemical cycles in an obvious sense, and organismal death -- which is often directly followed by ingestion and assimilation by other organisms -- in a less obvious sense).
Regardless of whether the abiotic sector can "deliver" the various fundamental resources necessary to life, negentropy import can only be accomplished when two conditions are met: 1) when organisms capable of assimilating resources exist, and 2) when the latter are present when and where the resources are available. To envision a state-space functionally connecting the biotic sector with its abiotic milieu, therefore, we must grant that evolution has produced organisms capable of both finding and processing the resources necessary to their individual maintenance as steady-state systems. This virtual truism -- and the even more straightforward idea that such resources exist to begin with -- must be taken here as given.
Another idea necessary to the development of arguments to follow is that obtaining and assimilating resources, especially food, requires outlay of energy. This investment leads to an immediate net increase in entropy levels within the biotic sector as chemical energy is converted to heat during the search process. The increase is then balanced, however, by the negentropy gained (imported) as the ultimate result of assimilation of foodstuffs. A similar costs-benefits framework can be used to understand the strategies associated with micro-habitat selection [6, 30, 31, 32, 33, 39].
The energy budget of an organism (or population thereof) may be understood to operate on two levels. When organismal function is viewed simply as a reflection of the internal equilibrium resulting when input balances output, steady-state conditions are implicitly recognized and the meaning of [[p. 237]] the energy expended cannot be extended to an evolutionary perspective. On the other hand, when the energy budget of the organism is taken to reflect a commitment to certain levels of interaction with the elements of its environment, a different kind of understanding becomes possible. With the reversal of frame of reference the energy expended can be looked on as a possible force driving change. Where an organism's activities contribute to any process ultimately leading to a net reduction in the amount of energy that need be expended later under analogous circumstances to obtain and re-assimilate a given resource, it follows that evolution within the overall biotic sector has occurred: the same amount of negentropy has been imported at a lower cost in entropy production. The implication is that biotic sector evolution proceeds as a function of the contribution of organismal activity to the development of a continually more efficient resource turnover process; i.e., one in which the modes of return become increasingly ordered.
The idea that refining turnover of environmental resources is related to community development toward steady-state conditions contributes fundamentally to the theory of ecological succession. E. P. Odum [83, p. 256-257], for example, states: "An important trend in successional development is the closing or 'tightening' of the biogeochemical cycling of major nutrients, such as nitrogen, phosphorus, and calcium. Mature systems, as compared to developing ones, have a greater capacity to entrap and hold nutrients, for cycling within the system." On first reading, the recursive view of change expressed in this passage sounds like a direct re-statement of points made in the preceding paragraph. Strengthening this impression is the fact that the physical environment and its changes have important effects on where and when community successional change will take place. There is, however, nothing within the ecological succession model that can be used to portray irreversible kinds of change at the global level. Successional cycles are described in uniformitarian terms alone, a constraint making the classical [[p. 238]] notion of the "climax" rather restricting [83, 87, 117]. The ecological climax idea leads to a steady-state interpretation of community organization virtually by definition, a position not compatible with the fact that population-level evolution must be proceeding even where (or if) local ecological equilibrium is approached. This only suggests that the "tightening" of resource cycles works at two levels: one involving the short-term integration of populations into quasi-stable, self-reproducing community structures, and another associated with a kind of change in organisms that leads in the long-term sense to increasingly efficient community structures. It does not suggest, of course, how to go about functionally interrelating these two levels of organizational influence.
The recursive nature of resource cycle development is also treated in the literature on biogeochemical succession [note, for example, references 19, 56, 80, 110, 112, and 127]. Related discussion, however, tends to either closely parallel that on ecological succession, or concern long-term kinds of change difficult to apply in detail to the study of evolution at the individual population level. To find a more adaptable understanding we must initially look elsewhere.
Every organism (or population thereof) acts as mediator in the general turnover of resources in its encompassing environment. Obtaining and processing resources requires an expenditure of energy, and, as suggested earlier, if there should occur from one turnover cycle to the next a general reduction in the amount of energy expended by a population to obtain a given resource, all other things remaining equal it follows that the overall system has undergone "evolution". Note, however, that we cannot conclude from this that evolution has occurred within that particular population. Before we can suggest how present ideas are related to irreversible change within individual populations, we must return for a while to discussion of biotic-abiotic sector exchanges.
Complex system function is often characterized in terms of feedback relationships. Huggett [54, p. 91] describes these as follows:
[[p. 239]]". . . The interplay of positive- and negative-feedback relations in a system can be subtle. Paradoxically, both types of relation can operate simultaneously to maintain the over-all stability of a system . . . homeostasis is that group of system-stabilizing relations which are characterized by positive feedback. Homeostasis may be thought of as all those relations which act to preserve a system by keeping it in steady state during its existence. Homeorhesis may be thought of as all those relations which act to preserve not a steady state but a flow process. . . "
In the set of relations depicted in Figure 1, the biotic sector is characterized as lying at the intersection of single (though highly generalized) positive and negative feedback processes. Positive feedback from the abiotic sector enters the biotic sector as a flow of (potential) energy and mass which fuels both life-sustaining and system-changing processes. This flow is "received" and redirected in as many ways as there are existing pathways within individual organismal structure. Recognizing this helps us to re-assess the classical understanding of the role of adaptations in evolution. In one sense, adaptations can be viewed as homeostatic devices. Again, when the energy budget of an organism is referred solely to its individual thermodynamics, a steady-state system is envisioned; the structural ends to such self-maintenance may be termed adaptations. On the other hand, when its energy budget is viewed as being committed to a routine of activity that contributes to functional interaction between itself and its environment, the adaptational suite of the organism can be construed as operating under a homeorhetic regime. Specifically, nonequilibrium conditions can be created if the organism returns mass and energy to the abiotic sector in amounts essentially equal to those received, but at different locations. (This is not the only way that disequilibrium per se between the biotic and abiotic sectors can be generated, of course. Macro-change in the abiotic sector environment itself can also produce such results, but in the absence of compensating biotic sector activity of degree greater than these, net system order cannot be increased. It is the matter of this compensation in excess of simple offset that [[p. 240]] we are concerned with here.)
With this picture of the two roles of adaptation, the aspatial biotic sector evolution model initiated earlier can be completed in outline. It is not difficult to view the historical development of suites of adaptations as mirroring negentropy increase within the system, and not much of a step further to connect the same process to the development of increasingly efficient resource turnover cycles: elaboration of structure has no purpose if not associated with elaboration of function, which in this case may be related to refining levels of exploitation of an ever-complexifying array of available resources. I therefore surmise that biotic sector evolution occurs as a function of the inability of abiotic-biotic sector interaction to reach equilibrium, or, more descriptively, as adaptation leads to the kinds of organismal activity resulting in a continuing net reduction in the amount of energy required to return vital resources to the same stage in a given cycle type. An important ingredient in the overall process has not yet been specified, however -- we now need to know what that activity is. Thus, what is it that actually keeps the members of each population system in continuing thermodynamic equilibrium as the abiotic sector changes in response to various kinds of historical inertia?
The answer, I believe, is movement through space and the spatial interaction that is part and parcel of that movement. A fundamental characteristic of living things is their ability to change location within their frame of reference. Such movement may be restricted to certain portions of a life cycle, but there is surely nothing alive that lacks this ability. Changes in location serve the immediate purpose of bringing an individual organism into physical reach of those items necessary to existence; in the longer term / larger scale sense, however, population-level locational adjustments may be viewed as acts which are necessary to / inherent in the continuation of inter-sector linkage in the face of the reality of ever-changing environmental conditions and bio- [[p. 241]] logical inertias. The equilibrium approached at any cross-sectional instant can never, of course, be maintained, as the particular suite of adaptations developing in response to one set of conditions will never be quite appropriate for dealing with any later conditions. (This is, in effect, a biotic sector-level generalization of the "Red Queen Hypothesis" [113].) We have named the biological interaction process corresponding to the on-going act of resolving this disequilibrium natural selection; the biological result of the process is the development of new responses to the sets of conditions encountered; i.e., new adaptations.
This aspatial characterization of the relationship between evolutionary process and form can now be complemented by a parallel spatial characterization. As just stated, disequilibrium conditions are potentially created when organisms return energy and / or materials to the abiotic sector in amounts equal to those received, but at different locations. In order to understand such disequilibrium producing spatial results parallelling what we already accept as biological evolution, it is necessary to postulate the existence of ordered change in spatial interaction patterns. Specifically, we should expect that the individual organisms's (or population's) operation within its behavioral space contributes to non-random change in the manner in which energy and materials are later made available again. Although this follows logically from earlier arguments, it turns out to be relatively difficult to actually prove it so. Evidence derived from studies of the habits of individual organisms can be of little help; the spatial and temporal frames of reference involved are simply too limited. Population-level studies appear to offer better prospects. Were range adjustments directionally random in nature, it seems that no net change in the overall state of the system could be possible: all instances of relative gain in system information levels would be balanced by relative losses elsewhere. Directional channelling of range change thus seems the only way that consistent gains can be made.
[[p. 242]] What I should like to explore in the remainder of this paper is the possibility that the net direction of organismal range adjustment on the terms presented may be predictable. This supposes that some set of underlying causal conditions generalizeable across populations exists, and that measureable surrogates for these can be isolated. In the discussion to follow, only the first half of this supposition will be examined; I have given the second matter an initial treatment elsewhere [104].
It is not difficult, as it turns out, to use ideas presented here so far to suggest a tentative model making such prediction possible, at least in theory. Simply, we should expect those areas where vital resources are being returned to availability at the most ideal rates to be those toward which ranges will most likely extend. It appears reasonable to suppose that selective forces will be greatest where resource cycling operates in the most suboptimal fashion; i.e., where abiotic sector constraints make maintaining thermodynamic equilibrium while functionally being "at the right place at the right time" to collect and process vital resources most difficult. (An obvious example of this kind of relation is offered by the irregular conditions of water supply in deserts and the many special strategies that desert plants and animals have developed to ensure successful reproduction when precipitation does occur.) As the selection process itself ostensibly consumes both time and energy, it follows that, on the average, those habitats demanding the most selection as a condition for occupation will be those occupied the most slowly (through in-dispersal) and removed from the most rapidly (under conditions of environmental change).
To develop this idea, we need first relate the dynamics of population range change to the supposed spatial variation in return rate characteristics of vital resources.
[[p. 243]] 4. SPATIAL INTERACTION AND EVOLUTION
The most fundamental spatial characteristic of a population is its distributional range. This will change through time as the population responds to various influences; distributional range, in fact, is as direct a correlate to the evolutionary history of a given population as is any trend of behavioral or morphological change within it, since range will appear as a delimitable pattern with the initial divergence of the form and disappear only with its extinction. The correlation between range and habitat, however, has in the past all too frequently been interpreted as a direct causal relationship. Limiting factor concepts better referred to individual-environment interaction have been used time and time again to defend the idea that species X appears to be restricted to a certain areal distribution. Whether this extension of individual level thermodynamics to the population level is legitimate is debatable, but use of the device does, at least, provide a reasonably straightforward portrayal of micro-ecological dynamics. In the state-space picture emerging there is a direct coupling of a positive feedback flow (materials and energy made available to the biotic sector as a function of their transmission through the abiotic sector) to a negative feedback response (morphology and behavior).
Recall, however, that the steady-state recognized above is a fiction in the longer-term sense. We know that the system, and its component populations, change irreversibly over time. I have already suggested how we might view this change at the level of biotic sector functions. To apply these notions to the modelling of individual population change in space necessitates some revision of the notion of environmental "control". Specifically, the environment must be interpreted as in some manner encouraging change rather than forcing constancy (or, metaphorically, as wielding a "carrot" rather than a "stick"). As a first step in this direction we can make use of the ideas of Maruyama [77].
Maruyama made an important contribution to General Systems [[p. 244]] Theory with his delineation of the concepts of "deviation-amplifying positive feedback" and "deviation-countering negative feedback". The following passage from Greer-Wooten [45, p. 17-18] introduces these ideas in a useful way:
"The fact that the second law of thermodynamics holds for an open system plus its environment, but not for the system itself, does not appear to have been sufficiently appreciated earlier. The 'steady state' is defined by the approach of minimum entropy production, and in fact at that time entropy is maximized -- subject to the conditions in which the steady state was attained. There is thus a continual tendency to the development of maximum entropy, given a certain structure and set of input and export relationships for the open system. Any changes in the environment will result in disequilibrium and the beginning of a new cycle.
Chorley describes such developments with changed imports as the open system tending towards higher levels of organization, becoming more ordered and heterogeneous. Such changes appear to be contrary to the second law, but Maruyama accounted for them by deriving important notions to describe open system change in general. If the exchanges of the system with its environment do not change markedly, then entropy production continues to be maximized up to the steady state. Such imports have been labelled negative entropy (or negentropy) and are also called 'information' and 'order'. However, the reactions of the system components to imports from the environment are also important in describing system change. Such system 'feedbacks' can be of two types: negative feedbacks (deviation-countering processes) maintain equilibrium situations, conditions that Maruyama called 'morphostasis'; in comparison, positive feedbacks (deviation-amplifying) influence system change, whether it be towards greater or less order. . .
One important conclusion that can be drawn from the discussion above is that in analyzing the dynamics of systems, the researcher should place more emphasis on flows (of energy, materials, or information) between components of the system, and the system and its environment, than on changed attributes of the elements."
In the above remarks a point is made that has special relevance for the present discussion. This is the notion that a system's response to imports from its environment may involve either deviation-countering or deviation-amplifying processes. It has already been suggested here that adaptation can be viewed as a deviation-countering process; accepting this idea allows us to maintain the classical eco-physiological truism that an organism must be adapted to the [[p. 245]] conditions imposed on it by its environment to persist there. As earlier stated, however, globally-nonrandom patterns of range change and the history of adaptational development seem to suggest a system that is not in equilibrium; that is, one that as a whole serves a deviation-amplifying regime. But we have seen that the ongoing development of new adaptations can be viewed as no more than a continuous change in: 1) the means by which ecological equilibrium is maintained (homeostatic view); or 2) the potential for initializing change (homeorhetic view). The process of change itself -- the continuing movement of the overall biotic sector away from equilibrium and in the direction of higher levels of order -- is at most correlated with the process of adaptation. Inappropriate interpretation of the nature of this correlation can only lead to confusion, as it is too easy to be mis-led into thinking that adaptational change (that is to say, deviation-amplification) involves population-level modifications that must always be portrayed as order-enhancing. Can evolution logically be viewed as nothing more than organic diversification, given what we know about the limited potential of highly specialized forms to generate the kind of variation necessary to continue the overall process? Overspecialization should be understood, as should any kind of adaptational change, as having resulted from a process of deviation-amplification, but where the operation of a process leads to results not capable of sustaining it, a system surely must be viewed as having moved in a direction of less order (in this instance, a temporary trend halted by extinction). It is not inconsistent, of course, that the results produced by evolution might fall along a continuum ranging from regressive to progressive. The point, however, is that this continuum distinguishes results only; it does not characterize adequately the basically progressive (irreversible) nature of the overall process. Wiebes [121, p. 243] has dubbed adaptation "the historical narrative of evolution". This characterization seems all too appropriate, as in the absence of clearly identifyable causal associ- [[p. 246]] ations, only "what's" can be characterized. All this leads to the suggestion (echoing the last words of Greer-Wooten quoted earlier) that it may be more beneficial to look at evolution as a process taking place between organisms than within them; that is, as a dynamic form of spatial interaction rather than adaptation.
Those who feel uncomfortable with the implied connection between adaptation and spatial interaction might be tempted to argue that the whole line of thought reduces to a "chicken or egg" circularity, but this is not the case. Spatial interaction does not follow adaptation or vice versa; rather, the relationship between the two is closer to that of form to function. From comments made earlier it may be inferred that range and range change can be related to spatial interaction in the same way. In the ecological sense (and with respect to the biotic sector), spatial interaction may be treated as the characteristics of spatial ordering of organisms such that thermodynamic equilibrium can be maintained between the biotic and abiotic sectors. We may move from this base to define spatial interaction in the evolutionary sense as a process of mass and energy exchange with the abiotic sector in which the amounts "returned" are about equal to the amounts "borrowed", but in which the location of return becomes increasingly ordered; i.e., as an active response to spatial variation in the abiotic sector's natural potential to turn over resources necessary to biotic sector function [see references 48, 51, 86, and 124 for more typical geographic applications of the term "spatial interaction"].
Integrating spatial interaction into the causal structure of evolution can be viewed as useful in several immediate respects:
1) To begin with, we are provided with means lending themselves equally well to either state-space or process modelling efforts. As part of a discussion of a nonequilibrium theory of biological evolution proposed by Wiley and Brooks [122], Wicken [120, p. 442] has remarked: ". . . internal ordering depends on a system's ability to export entropy to its [[p. 247]] environment. . . . The virtue of the thermodynamic approach to evolution is its ability to connect life ecologically to the rest of nature through shared matter and energy flows; denying the ecological component of evolution, or the influence of ecology on development, badly weakens (their) thermodynamic base." Wiley and Brooks's theory has also been criticized on other counts [9, 71]. Nonetheless, they continue to defend it [12, 123]; regarding the matter of the effect of ecology on evolution they only claim to be "rejecting ecological determinism" [12, p. 94]. A similar stance is taken here, as the "forcing function" of the environment is viewed as implemented at the community, rather than individual, level. The environment can, I submit, effect direct control over what kinds of spatial interaction processes operate among organisms but not, in the terminology of Brooks and Wiley [12, p. 93], over the way "the phase space defining the maximum number of microstates which the evolving lineage could occupy" changes with time (since this potential is, as Brooks and Wiley would argue, a function of the particular constraints and potentials developed over the line's own history, as "summarized" at any given time within its gene pool). Carson's [17] "outside variables" (here, abiotic sector provision of vital resources) may thus be interpreted as defining the state-space within which organisms find themselves in the immediate sense, but not in such a fashion as to subvert the "individuality" of development of any given evolutionary line. (Later, I will make parallel observations, as is logically necessary, regarding the relationship between the individualistic hypothesis in ecology and the dynamics of range change.) This overall causal structure has the obvious advantage of lending itself to ecological state-space description in which the controlling variables may also be understood to produce a kind of change that need not be viewed as "ecological determinism" in the Brooks and Wiley sense.
2) The present portrayal of the complementary -- but still entirely separable -- roles of spatial interaction and adapta- [[p. 248]] tion also solves outright the philosophical dilemma attending the awkward position that evolution involves a process (adaptation) yielding structures (adaptation) of non-independent definition [11, 35, 43, 47]. As Lewontin [70, pp. 237-238] has put it, "The process is adaptation and the end result is the state of being adapted. . . The problem is how species can be at all times both adapting and adapted." When evolution is understood as the disequilibrium inherent in biotic sector / abiotic sector spatial interaction (and not "the process of adaptation"), the homeostatic, "ecological", role of adaptation can be accented as a "result" to provide a straightforward causal picture devoid of circularity and attending logical difficulties. In this view, adaptations are regarded simply as the structural attributes that mediate energy degradation, or that, as Wicken [120, p. 440] puts it, "provide a means by which potential energy can be converted to thermal entropy and released to space."
3) Further, re-interpreting evolution as a spatial interaction process provides a response to the complaint that the study of the "evolution" of adaptations (i.e., phylogenetic studies) reduces to idiographic "narrative" [41]. Particular adaptations are still regarded, of course, as arising in response to one-of-a-kind combinations of environmental and biological circumstances; given genetic constraints on the way change must take place, we should expect the exact manner in which potential energy is converted to thermal entropy to remain individually unique with respect to each population. This understanding -- focusing on the homeostatic function of adaptation -- resists any systematic biological interpretation beyond the identification of when and where each novelty arose (and the narrative sequencing of this information with all other such information). But when the homeorhetic function of uniqueness (adaptations) -- spatial interaction -- is emphasized, such criticism is rendered moot. Following this interpretation makes it possible to think of irreversible processes as leading to more than the unique structures we call adaptations; specifically, to standing interaction [[p. 249]] patterns interpretable on normative grounds as well: in the biological sense, for example, as competition / natural selection, and in the spatial sense as distribution patterns (in effect, an answer to Eldredge's [26] complaints regarding the "just so" nature of much of descriptive biogeography). In this view, it is the properties of spatial interaction that evolve, not the organisms themselves.
4) A functional application of the perspective is provided by a possible reinterpretation of the "saltationist-incrementalist" debate that extends back to Darwinian times; that is, whether evolutionary change proceeds in sudden starts and stops or gradualistically [see references 27, 37, 42, 90, and 91 for representative discussion]. The basic problem is that the fossil record provides no proof for a gradualistic kind of evolutionary progression despite the fact that this is what classical selection theory calls for. The emphasis of this discussion, however, must be viewed as somewhat misdirected when examined in the light of ideas set out here. Note that the fossil record is ordinarily used to document changes in the structure of individuals; i.e., in mode of adaptation. I have been arguing that changes in bodily form / behavior can be held to represent either the results of evolution, or a kind of potential for evolution, but not the interaction process constituting evolution itself. Regardless of whether saltation might describe the manner of sequential unfolding of adaptive assemblages, it may or may not describe the way changes in the spatial interaction structure involving the biotic sector take place.
This realization helps us to identify a fundamental problem in the way saltationist / gradualist discussions have developed. The gradualism point of view (e.g., as held by Darwin, Wallace, Mayr, and Simpson) is fundamentally an externalist's approach to evolution; through it natural selection becomes largely a matter of environmental selection (whether the latter be specified in physical or biological terms). Those following the saltationism line, on the other hand, have implicitly fallen into an internalist's mode of [[p. 250]] thinking: that evolution is regulated by potentials and constraints acted upon by the environment [note the arguments posed in references 12, 43, 44, and 122]. It has been the mistake of the gradualists to synonymize evolution with observable changes in form of organisms over time; this view leads to the "adaptation yield adaptation" circularity philosophically, and to the empirical contradictions of the fossil record. Internalism-based arguments denying gradualism, on the other hand, are equally short-sighted. It can hardly be believed that environmental variability and change does not have important influence on the fact that characters are selected, regardless of whether population-specific constraints are also involved.
It seems to me that a comfortable resolution to the overall issue is not possible at present; two problems are outstanding. First, as Gingerich [37] points out, philosophical arguments notwithstanding, only empirical studies can determine what the actual patterns of divergence have been, and we do not have enough relevant data at hand. But note that even this will not wholly settle the matter, because, as just mentioned, it may not follow that a particular pattern of divergence is underlain by a corresponding routine of change in spatial interaction patterns. In short, a better philosophical position than that offered by "internalist / externalist" and "saltationist / gradualist" alignments is also needed. Such alignments at best mis-specify the causal structure involved; there are no "internal" and "external" factors in / to an evolutionary process that takes place between things, not within them. Despite the fact that his analysis focusses on internal factors in evolution (thus perpetuating current biases), Waesberghe [114, p. 26] comes to similar conclusions: "To an alternative model evolution is the saltatory origin of new taxa, prepared by a gradually improved ecosystem of interdependent external and internal factors."
In the four discussions preceding, an underlying theme can be perceived. I would argue that the classical natural selec- [[p. 251]] tion causal model is incomplete, because recursive change in the biotic sector is most fundamentally guided by selection producing more efficient means of turning over material resources (spatial interaction), as opposed to "fit" individuals and population. This in no way denies that the latter kinds of results might also be produced (indeed, we should be able to deduce this); they are, however, viewed here rather as the immediate by-products of a more general, and continuous, process. Ignoring this prior causal structure is likely to lead to difficulties of interpretation when we attempt to confront the longer-term implications of immediate function (for example, the earlier mentioned relationship of "fitness" to episodes of divergence leading to overspecialization and extinction). This structure -- involving the dynamic interrelationships among distributional changes in populations, their adaptive capabilities, and environmental input -- might be described in general terms at this point with the aid of the following words of Kenneth Boulding [10, p. 13]:
"Another phenomenon of almost universal significance for all disciplines is that of the interaction of an "individual" of some kind with its environment . . . each of these individuals exhibits 'behavior', action, or change, and this behavior is considered to be related in some way to the environment of the individual -- that is, with some other individuals with which it comes into contact or into some relationship . . . The 'behavior' of each individual is 'explained' . . . by certain principles of equilibrium or homeostasis according to which certain 'states' of the individual are 'preferred'. Behavior is described in terms of the restoration of these preferred states when they are disturbed by changes in the environment."
In the present context the "individual" may most conveniently be taken as a population, with "behavior" synonymously being range (spatial) change and adaptation. These are viewed as twin aspects of the evolutionary process through which populations contribute to the ecosystem's general progression toward a steady-state material turnover regime. Of particular interest here is the spatial interpretation of the "behavior" concept portrayed above. Emphasizing this aspect will allow us to regard the "preferred states" of organisms / populations as being evolutionarily transient and [[p. 252]] individually unique in a biological sense (adaptation), yet still open to normative interpretation (as distribution patterns exposing generalizeable states of spatial interaction among populations and their environment).
5. THE ANALYSIS OF SPATIAL INTERACTION
Philosophical ramblings aside, there seems little reason to attempt to further complicate our understanding of the causal basis of evolution unless it can be shown that doing so leads to possible advances in methods of practical study. The ideas that have been introduced here so far are largely heuristic; we now turn to a consideration of how to use them to study actual biogeographical and biological processes.
I have mentioned several times that adaptation and range change represent parallel processes within the present evolutionary model. To operationalize the model, we must show that spatial interaction per se is an analyzable "commodity". As sketched so far, the concept promotes a view of evolutionary causality focusing on the community level of organization. To make this understanding operationally useful (i.e., to promote normative study), we need to suggest how communities "evolve" in response to spatially-varying levels of efficiency of abiotic sector provision of necessary resources. This will require showing how population range change (and associated integration into community structures) can be influenced by this exogenous factor in a manner that is predictable across populations.
6. SPATIAL INTERACTION IN THE COMMUNITY CONTEXT
While range change is often examined from the perspective of within-population processes [e.g., 3, 89, 97, 111], it is clear that it does not take place in the environmental sense in a void: distributional changes also reflect constraints and opportunities served up by local community organization properties [13, 28, 45, 65, 94, 126]. There is no reason, in fact, why the setting itself cannot provide the point of departure for range change studies. This approach is particu- [[p. 253]] larly useful when one becomes interested in normative modelling, as a kind of flexibility lacking in population level perspectives may be exploited. From an analytical standpoint, it is difficult to view a species population as other than an ecological or a historical entity, because the species is a relatively individual and "irreversible" entity in both space and time [7, 36, 95]. The historical element implicit in the notion of the species is its genetic relation to other species (that is, its relative position in the life hierarchy), not to the history of the ecological system continuously sustaining it. Little information regarding the geographic location of the particular community in which an organism happens to find itself is directly stored in the form of adaptations (one immediate reason why physiological ecologists have contributed relatively little to evolutionary theory). Communities, on the other hand, are neither rigidly delimited -- or even, perhaps, delimitable -- in space and time, nor express much of anything about their "genetic" relationship to communities elsewhere. Nonetheless, like species, their characteristics can easily be linked to ambient environmental conditions, and they do exhibit a historical side: the pattern, over time, of assimilation of species populations into them.
This is a useful association. It affords a means through which history can be viewed in terms of spatial interaction instead of irreversible outcomes (phylogenies): community change may be portrayed as reversible in the sense that spatially it may involve both subtraction and addition of forms over time. Through range change episodes, given populations may extend to, or withdraw from -- or do both many times -- a particular community structure. The kinds of intra- and inter-community distribution patterns emerging from such spatial interaction are inherently interesting, because areal patterns per se are not population-specific and therefore potentially lend themselves to normative modelling approaches. All populations are members of communities ipso facto and contribute to the non-population-specific resource turnover [[p. 254]] processes mediated by community organization. The key issue, however, is whether the reverse can be demonstrated: that the turnover characteristics in space and time of some one (or more) vital resource force populations into correlated patterns of integration (as community structures). Biologically, the associated assimilation process will include a bewildering array of adaptive changes dependent on population-specific histories, and will be difficult to link, across all populations, to individual abiotic sector forcing functions. The spatial character of assimilation, however, will be directly evident in changing distribution patterns, which might be interpreted more easily: for example, as an innovation diffusion process fueled by spatial variation in the characteristics of availability of one or more fundamental abiotic sector-mediated resource. [Human geographers have taken considerable interest in the modelling of such relationships; see, for example, references 4, 14, 50, and 101].
Supposing that it is useless to attempt a definition of community grounded in the evolutionary histories of the species populations making up communities, there can likewise be no prior meaning attached to the specific suite of phenotypic expressions associated with them. This is not to say that general classes of adaptational strategies (adaptation to extremes of cold, heat, moisture, etc.) cannot be linked to particular kinds of habitats or community structures, but instead to remind us that the genetic means to such ends are in a historical / phylogenetic sense idiosyncratic; that is, taxon-specific (an aspatial re-statement of the individualistic hypothesis). We are otherwise forced, it seems, to believe that particular lineages and strategy types come together necessarily to produce the community characteristics we witness, a teleological viewpoint that is difficult to reconcile with either the empirical evidence to the contrary presented by supporters of the individualistic hypothesis, or the normally stochastic characteristics of spatial interaction processes.
[[p. 255]] Conservative treatment of the community assimilation process thus demands we start with the proposition that communities are in some sense accidental structures that evolve as a simple function of the particular populations that happen to arrive and become integrated into them. This view, in fact, closely corresponds to current framings of "community" as a "concept" rather than a prior reality [see 118, and 119]. The individualistic view is well summed up by Whittaker [119, p. 327]:
"Species are distributed 'individualistically', each according to its own way of relating to environment . . . Species do not fit naturally into groupings that correspond to community-types and are discontinuous with other such groupings . . . Community-types are not natural but arbitrary units in the sense that their extensional definitions are strongly influenced if not wholly determined by phytosociologists' choices of the characteristics by which communities are to be classified . . . further . . . not only species but also groupings of species . . . show relative independence of one another, and may be differently combined into particular communities."
Whittaker thus argues that community structure will develop as an un-predetermined function of the collective adaptive flexibility of those populations coming together. This position seemingly casts long-term community-level change as a kind of chance drift phenomenon analogous to the one now argued to take place at the genetic level [59, 60, 61]. Unfortunately, there are problems with carrying this interpretation very far. Communities may be viewed as areally-limited portions of the biotic sector. Like the biotic sector in general, they capitalize on the abiotic sector's resource turnover capacities. A population's exploitation of available potential energy sources is not a free ride, however -- it is accomplished only at the price of irreversible genetic change that can contribute to limiting its term of existence by reducing its ability to constructively respond to later environmental changes. Van Valen's "Red Queen Hypothesis" [113] in fact, seems to be valid whether populations continue to change or not -- subject to the conditions of environmental change surrounding them. Genetic invariance over time need not be fatal, as long as the environment remains constant.
[[p. 256]] To one extent or another, however, environments do not remain constant; as a result, "evolutionary deadwood" is continually being removed from the stage. Meanwhile, range change episodes are contributing to the development of new kinds of spatial interaction, permitting the biotic sector to continue its overall process of complexification through diversification. Now, then, how does all this reflect on the individualistic hypothesis? Fundamentally, it suggests that communities are prior entities, but for reasons that have nothing to do with the characteristics -- distributional or adaptive -- of the populations making them up. (Note that this in turn suggests that the empirical evidence of individualistic distribution cited by defenders of the individualistic hypothesis is entirely irrelevant, characterizing as it does the (ecological) results of a (-n evolutionary) process rather than the process itself.) While it may well be true that species are distributed "each according to its own way of relating to environment", this in no way argues that these "ways" cannot be forced into correlating with one another in a spatial sense. Communities may not be "prior" entities, but the environmental envelopes sustaining them are, at least effectively. The nature / degree of this exogenous control can almost certainly be viewed as varying over space; accordingly the amount of information in the physical setting potentially exploitable by living things should also vary. As "negentropy-importing machines" [99], organisms can be expected to seek out and exploit such sources of information. In the aspatial sense, this exploitation has been viewed straightforwardly -- in the classical manner -- as "evolution"; i.e., the historical development of effective adaptive structures. An appropriate understanding of associated spatial change events, however, necessitates a somewhat greater degree of abstraction.
When we associate biotic sector aspects of evolution with non-random spatial change, we cannot view range adjustments as being independent of the pattern of spatial variation of abiotic sector potential information available. As suggested earlier, it is reasonable to believe that, on the whole, [[p. 257]] ranges will tend to extend (or contract) in directions of higher environmental information levels. Both overall system evolution and individual population change will accompany this directional channelling of populations. The first will take place as community spatial interaction structures evolve; i.e., as patterns of material exchange between the biotic and abiotic sectors develop which permit change in the direction of steady-state conditions. Selection on a by-population basis associated with these developments will produce the range of adaptive innovations needed to maintain the overall structure. Importantly, there will be two nonpopulation-specific spatial components associated with the latter process.
Even if we interpret range change as a net "up-gradient" movement response to spatially-varying environmental potential information levels, the actual process should operate under the influence of orthogonal forcing vectors. Ranges are actually most likely to extend with greatest rapidity in directions of similar environmental potential information levels -- "along contour", as it were -- because such movement will not be slowed pending the selection of new traits. Moreover, the stronger the spatial "information gradient" involved, the stronger will be the tendency to expand along contour rather than perpendicular to it. This argument applies, I believe, to a fairly wide range of spatial / temporal conditions.
Range expansion of the "along contour" variety will be limited ipso facto by the size of the corridor involved (and / or the patchiness of the environment); once this is filled, any further population increase will force a "spillover" of individuals across -- and most rapidly up -- gradient. Selection favouring those individuals with the flexibility to exploit new conditions will then lead to adaptive change [111]. The degree and rate of this change will probably not be generalizeable across populations, but this should not stop us from proposing other possible associations that very well might be. For example, where the operating information gradient is very slight or absent, we should predict that direc- [[p. 258]] tion of range change will tend to be largely random -- at least as compared to those places where a well-defined one exists. Where potential information levels are very high, we might expect adaptations to emerge in a nearly "drift"-like fashion: the constraint of needing to be "in the right place at the right time" minimized, morphology and behavior should develop as a function of within-population (historical) limitations (variation) alone. Each of these general classes of studies -- on: 1) range change as related to the areal distribution of potential information gradients, and 2) spatial variation in selection regimes as related both to gradients and absolute levels -- can involve a wide range of possible non-population-specific hypotheses [104].
Depending on one's level of attention, therefore, an idiographic or normative stance can be taken regarding whether populations are individualistically distributed. That each responds to unique sets of immediate requirements need not be debated; of greater interest, I submit, is to what degree their properties of biological change are spatially correlated through time. In the latter view, communities are dynamic spatial interaction structures that evolve as populations are channelled into them in a nonrandom fashion dictated by prior environmental constraints. Degree of community "individuality" thus becomes a function of the degree of channelization involved, and how long it has been going on. It is not significant that the distribution boundaries of individual populations do not neatly delimit community structures, because in the spatial sense the latter are referable only to areal variation in the relative "drawing" (or "repelling") power characteristics of abiotic sector-level potential information levels (and not the actual limits of distributions themselves, which at any given time only serve to indicate the direction of preferred fronts of change across this field). Some further attention will now be given to this presumed channelization process.
One of the most common ways to relate exogenous controls to the character of community interaction patterns is through [[p. 259]] the twin concepts of density dependent and density independent feedback. The former involve selection factors whose effects on populations vary with population density [64, 82, 107]; the latter, factors whose effects do not, and that therefore are proportional to density [1, 24]. Climatic influences on selection are usually considered to be of the density-independent type, whereas more strictly biological phenomena such as competition, predation, and disease tend to be density-dependent in their selective capacity.
The application of these concepts [and the related r- and K-selection continuum notion: see references 57, 73, 92, and 111] has implicitly forced evolutionary ecologists into thinking that selection controls fall into classes only one of whose elements are exogenous to the community interaction structure. Although this approach makes it easier to appreciate the dynamics of individual populations, it is not easily relatable to the idea that resource turnover processes develop as recursive interactions between the biotic and abiotic sectors. A simplification relevant to such understanding should now be noted. In the context of the resource cycle model developed here (i.e., Figure 1), density-dependent and density-independent types of selection processes cannot be contrasted in the usual way, because all exogenous influences on biotic sector activity are exerted by abiotic sector-originated transfers. As defined earlier, the biotic sector is comprised of organismal "islands"; all imports / exports to / from each organismal unit involve crossings of the biotic-abiotic interface. The importance of emphasizing this derivative of the present model will become apparent shortly.
Analysis of the forms of exogenous control on community structure is also featured in the community de-stabilization literature. There appear to be two major accents in this: the more abstract notions of community stability and resilience, and the characterization of stressed ecosystems. The first subject cannot be satisfactorily reviewed here without pulling us too far off the track [see references 52, 53, 69, 75, 78, and 109]. Since the concept of stress is directly [[p. 260]] relevant to the present discussion, however, the following passage is reproduced [29, p. 50] in an effort to indicate how it has been treated historically:
"Stress as Cause
According to this approach, stress acts as an independent variable, external to the organism, being a stimulus or input which causes strain. This approach depends heavily on analogy with Hooke's Law . . . According to Levitt, stress is any environmental factor which is potentially unfavourable. The evolutionary response of organisms has been selection for avoidance or tolerance of the strain.
Stress as Effect
According to this approach, stress acts as a dependent variable, internal to the organism, being a response or output which is caused by some factor that is usually identified as the stressor. One of the best-known formulations of this approach is that of Hans Selye. His autobiographical account of its development emphasizes the notion that stress is the response of an animal to an external stressor. Furthermore, it is claimed that the response is physiologically non-specific and does not depend on the nature of the stressor. The stress response represents a universal pattern of defence, a mechanism to preserve the animal's integrity. . .
The predominant formulation of stress as effect is based upon performance. An organism is in a state of stress when some measure of its performance falls below par. This formulation can be traced to Liebig's law of the minimum or to Shelford's extension of it, the law of toleration. This tradition is basic to much of the experimental work in comparative animal physiology and agronomy. It recognizes that stress can be induced by environmental factors which are either above or below the optimum range. As no species encounters in any given habitat the optimum conditions for all of its functions, performance can be enhanced, as well as diminished, by manipulation of the environment. E. P. Odum et al. have referred to this effect as the subsidy-stress gradient. This effect is not confined to enrichment alone; impoverishment results in a subsidy when an environmental factor in excess is restored to the optimum range. . . "
The analysis following these words proceeds to a treatment of stress as both cause and effect. This is also a useful direction to take in the present discussion. To complete our "disequilibrium model" here, we need to identify a way in which the rate / pattern of provision of some vital resource(s) influences the development and operation of community structure in a manner forcing a deviation from the drift-like con- [[p. 261]] ditions portrayed through the individualistic hypothesis. This influence, which I shall term "stress", will be expected on the basis of earlier remarks to be linked to two kinds of complementary results: 1) a biological result, manifest at various organizational levels as community, population / demographic, and adaptational (morphological, physiological, and behavioral) structures and changes in same over time; and 2) a spatial result, manifest as distributional patterns and the way these change over time.
Let us imagine a commodity necessary to most aspects of biotic sector function that is essentially ubiquitous and unlimited in space and time (atmospheric oxygen perhaps represents the closest real world approximation). Such a resource can neither be a progressive selection factor nor an influence on distribution, as it will not be competed for and will prescribe no ordered patterns of interaction with elements of the environment.1 On the other hand, any vital resource that is available in potentially useful amounts only part of the time and / or in some places is by definition limited and can be understood to result in the latter kinds of influences. This notion could lead us back to natural selection and the biological structures produced thereby, a subject we need not dwell on here. The associated spatial interaction structure, however, is a matter of central interest.
It is apparent that any resource vital to all forms of life but available only part of the time and / or in some places will dictate certain spatial strategies of existence on the part of living things. Specifically, they will need to apportion a significant part of their total energy budget to the development and operation of means of being "in the right place at the right time" to obtain and conserve the resource. Such behavior, whether actively or passively initiated, will necessarily lead to the development of non-random movements through time and space (for sedentary organisms the patterns will devolve as spatially-varying rates of successes and failures of individual reproduction over time). I interpret [[p. 262]] this deviation from random movement in time and space to represent a direct mapping of the effect of stress on the system. The greater the stress (i.e., the more stressful the rate and spatial pattern of provision of the vital resource(s), the more non-random the kinds of movement (i.e., the more correlated with other movements) we should expect.
Such non-random patterns of direct interaction between the biotic sector and the abiotic sector should extend to the pattern of interaction among organisms. An obvious example is the well-known fact that in times of drought, carnivores often hunt near the waterholes their prey frequent. Commensal and parasitic relationships provide another, as do the various kinds of mimicry and protective coloration devices. In the first two cases, the locations of individual organisms become tightly linked; in the second two, locations of individual populations.
Given the appropriate operational model of the generalized influence of stress on community structure and function, I see no reason why it should not be possible to develop simple measures of stress that can be used to compare abiotic sector potential energy levels at different locations. It should be emphasized that it is not contradictory that the regimentation of spatial interaction patterns in communities might be equally extreme in very different kinds of environments, though manifest in different specific characteristics of nonrandomness. We find, for example, equally extraordinary adaptations in desert and tropical rainforest organisms, and can conclude that equally extraordinary selection regimes must have led to such results. It may be complained at this point that there is, however, a considerable difference in the kinds of selection regimes leading to the types of specialization associated with each habitat. The evolution of desert lifestyles seems to be dominated by physical environment constraints [1, 20, 49, 98], whereas the adaptations of tropical rainforest forms are more often biologically interrelated (involving the finer niche splitting [62, 72] associated with, for example, mimicry, mutualism, parasitism, [[p. 263]] canopy partitioning, etc.). This criticism, however, is irrelevant to present arguments. All that is posed here is that the (stress-imposed) degree of ordering of spatial interaction might be the same across different habitats. There is no reason to believe that this cannot be so, as it is known that very different ordering processes can produce different structural results of equally high information content [34, 67, 100]. Evolution may be a phenomenon evidenced by information storage, but it is enacted through information exchange. Stored information may assume a virtually infinite number of forms, and in the long run there is limited usefulness in attempting to describe them all. On the other hand, the characteristics of information exchange are likely to be generalizeable in the context of resource cycling, because the most fundamental limiting resources (especially water) are both equally important to all life forms and little changed by any particular form's use of them. The more the properties of cycling of such resources are arbitrarily split up, the more we lose the ability to identify influences on community structure that can be generalized. A good example of this problem is provided by the density-dependent / density-independent division. In related discussions, we are invariably trapped into focusing on the differences between the adaptational suites of populations (and associated macroinfluences on their development) at different locations; this overemphasis on uniqueness of kinds of result neglects the fact that regardless of locational history or the history of the populations involved, in all situations there are flows of more fundamental elements that need not be treated idiographically.
At the same time, of course, there is no reason why we cannot relate these flows to adaptations per se. Inasmuch as adaptations are portrayed through this view as correlates to the "geometry", as it were, of spatial interaction, wherever the exogenous conditions specifying the form of that geometry are similar, we should expect analogous suites of adaptations to develop. This makes the interpretation of large-scale [[p. 264]] convergent / parallel evolution [of the type documented in references 74, 81, and 88], for example, relatively straightforward.
Given the spatial version of the community interaction matrix [68] described above the following general model relating range change, exogenous controls, and community structure can be summarized. Community structure will develop as a function of the degree (and kind) of nonoptimality of rate and magnitude of provision of vital resources through the abiotic sector at a given location. The greater the exogenous stress exerted, the greater will be the degree of spatial ordering of populations (and their interactions) involved. The degree of stress and resulting nature of the community interaction matrix will also of necessity affect rates of in-dispersal of populations, since the latter will have to evolve behavioral / morphological structures allowing them to take part in the particular pattern of spatial interaction dictated by that stress. Where the stress is high, ordering will be great, and penetration of the structure by new populations will be more difficult because more selection will be necessary to produce the characters necessary to fitting into that order. Thus, rate of assimilation of populations into a given community will be a function of the degree of stress imposed upon the latter.
This deductive argument for the nature of the constraints on the community assimilation process can be strengthened by appealing to an argument analogous -- and perhaps complementary -- to that used by proponents of the neutralist view of evolution [58, 59, 60, 61]. As defined by Kimura [59, p. 208], the neutral theory "asserts that the great majority of evolutionary changes at the molecular level are caused not by Darwinian selection but by random fixation of selectively neutral or nearly neutral mutants in the species." The theory further states that under such circumstances, random drift will set in with respect to relative allelic frequencies, "so that most polymorphic alleles are maintained in the species by the balance between mutational input and random extinction [[p. 265]] (or fixation)" [59, p. 208]. With respect to community evolution, we might expect a similar process involving the assimilation of populations to take place. Where extra-biotic sector direction is absent (again, where there are optimum rates of resource provision), the addition of populations should occur under a simple drift-like regime. Organismal change will then take place as a function of: 1) the development of chance associations between co-existing forms (in turn depending on the historical constraints on adaptability built into the gene pool of each -- the Wiley and Brooks model); and 2) the constraint on such development that it not reduce system-wide resource turnover efficiency. When prior restrictions are added, however, a more ordered regime of selection will result in which phenotypes with particular adaptive potential are selected for: the potential to take part in the degree and kind of prior non-random interaction specified by the external stress. In general, it can be supposed that a continuum of kinds of ecological / evolutionary balances among rates of population integration ("fixation"), speciation ("mutational input"), and extinction within communities will develop that will depend on local stress conditions (and changes in these over time), and not, at least in the first instance, on the particular populations involved. (One supposes on this basis that the neutralist view should be modified to take into account long term changes in molecular numbers and balances; Hutchinson [56] has produced a related discussion. Particularly relevant would be the trend of increasing organic exploitation of oxygen over geologic time [19, 112]. From this point of view, a strictly "neutral" kind of molecular evolution would be possible only under conditions of stable turnover rates of all relevant basic building blocks.)
The degree to which community function and change operates under exogenous stress is potentially a measureable quantity [see reference 5]. Moreover, relevant data permitting, it should be possible to identify how this degree of stress to community development varies over geographical space. This [[p. 266]] leads to the possibility of hypothesis testing. Again, if community structure otherwise develops as the random situational integration of populations (the extreme version of the individualistic hypothesis), it can be adduced that for any given population initially entering into that structure (or withdrawing from it), the ease with which it will do so will be due to the degree of constraint on community drift. This is a testable hypothesis, because it suggests that direction of range change will tend to be toward those places that are least stressed, a proposition that can be investigated through various kinds of analysis of the cumulative patterns of actual organismal distributional ranges as these relate to such stress.
7. DISEQUILIBRIUM AND EQUILIBRIUM EVOLUTION
Figure 2 resembles Figure 1 in its depiction of the abiotic and biotic sectors of the earth's surface system, but is more explicit in its inclusion of additional feedback loops, especially that expressing the posited role of spatial interaction in the system's evolution. In earlier discussion, it was advanced that nonrandom movement through space accompanying the day-to-day maintenance of an organism's individual energy budget increases net system information levels by cumulatively feeding back to reduce energy output demands on the biotic sector in the long-term sense. In Figure 2A, the homeostatic function of adaptations is portrayed. Total energy input and output to / from each sector balances per time period considered. Note the addition of an inferred (and from a strictly physiological point of view, largely irrelevant) loop depicting flow of energy and resources (potentially) available to the biotic sector, but not used by it. In Figure 2B, the homeorhetic function of adaptations is portrayed. Through this understanding, we may adopt an evolutionary perspective in which organismal function leads to spatial interaction and non-random mass redistribution. The potential energy added through this redistribution is recursively "used" to continually reduce that portion of the flow of
[[p. 267]]
Figure 2. Expanded state-space (A) and process (B) views of earth surface system organization, per Figure 1 [after 104, p. 99]. Note that double lines indicate paths of both mass and energy; single lines, energy alone. See text for discussion.
[[p. 268]] energy and resources available to the biotic sector that ends up not being used by it (a process of worldwide resource cycle "tightening" amounting to net stress reduction, and corresponding to a general movement toward steady-state turnover conditions).
What does this general picture of the nature of surface change permit us to say about the longer-term development of inter-sector equilibrium conditions? Earlier, I alluded to some inconsistencies resulting from interpreting irreversible biological change as either a dynamic equilibrium or a disequilibrium phenomenon. We are now in a position to remark on this problem. Assuming, as has been argued, that spatial variation in the availability of some extra-biotic sector resource or resources acts as a "carrot" to which the spatial distribution of organisms dynamically responds, irreversible change within the biotic sector will proceed as a disequilibrium process as long as resource cycles continue to tighten at a global level. At some point, however, recursive abiotic-biotic sector interaction will be unable to effect improvements in efficiency of turnover: variability inherent in the system's overall internal operation and relation to longer-term cosmological processes will achieve a balance with the biotic sector's capacity for exploitation. Otherwise put, natural rates of range change will no longer be able to reduce the amount of potential energy inherent in abiotic sector conditions lost faster than the latter's own changes increase that amount.
This does not mean, however, that we can conclude biotic sector change will come to a halt when the point of inter-sector equilibrium is reached. Instead, change under conditions of dynamic equilibrium will be established. Though net world rates of resource turnover will remain constant, range change, speciation, and extinction should continue; local environmental information gradients will still be responded to, though local spatial / temporal "information gains" will be offset by losses occasioned elsewhere by local spatial / temporal environmental deterioration.
[[p. 269]] There is yet one more matter to be considered regarding these speculations, and it is interesting enough, I think, to justify my bringing up this whole matter of global equilibrium relations again. It seems safe to assume that the genetic / morphological / behavioral flexibility inherent in populations varies greatly. Accordingly, the overall degree to which the spatial distribution of stress channelizes population range change should also vary across populations. For adaptively very flexible populations, the deterministic influence of spatially-varying stress levels will be minimal. As in the case of absence of exogenous stress, the result should be adaptive drift of a non-irreversible type (i.e., of a type not referable to any aspect of abiotic sector-mediated spatial interaction, and therefore not constrained to take part in the overall movement toward steady-state conditions). Perhaps this is what accounts for the semi-"reversible" properties of differentiation within far-ranging genera such as Felis and Canis. Though specialization returns for a population the immediately useful result of stable ecological associations (i.e., integration into particular community spatial interaction regimes), there is also a potential longer-term advantage to resisting such irreversible associations. When local environments change rapidly, generalist forms can simply withdraw, if necessary, and be reabsorbed into surrounding populations. Forms highly specialized for taking part in particular spatial interaction patterns are less likely to be able to do this; as a result, we can expect generalist populations to become increasingly dominant over the later course of evolution, and especially so where stress conditions have varied greatly over time.
Identifying this trade-off makes it easier to suggest a possible non-deterministic context for human cultural evolution. The original Darwinian position on cultural evolution [23] -- since revitalized by the modern sociobiology school -- was that it could be treated as a simple logical extension of the natural selection model. This notion has not always yielded the most profitable applications -- note in this [[p. 270]] context the excesses of the environmental determinism and eugenics schools. Darwin's colleague Alfred Russel Wallace, on the other hand, demurred at an early stage [115] that natural selection could not be used to explain the development of the higher faculties, which he argued were unnecessary for mere existence and therefore contradicted the inherently conservative position of natural selection on the possibility of "overadaptation". While I do not wish to go on record as supporting Wallace all the way on this issue, it seems to me that his position -- to some extent even his eventual invocation of "supra-physical" causal powers -- in the end provides a context channelling inquiry in a more productive direction. Note that in the present model, global-level change under dynamic equilibrium conditions is essentially change undirected by "physical" spatial constraints; i.e., the "tyranny of space" has been lifted. This is not to say, however, that such change must necessarily be "directionless". If it is not, in fact, the environmental / biological determinism issue might finally be put to rest: cultural evolution could be interpreted simply as a supra-disequilibrium process not directly shaped by the restrictions of physical space. In recent work [105, 106] I have presented a general model of spatial structure that suggests how this can be so. I intend to prepare a separate paper dealing with the relationship between social evolution and this model; for the present I will only comment that I believe Wallace's restriction of the possible role of "physical / biological" space in producing organic change was an important step forward that has not, like so many of his advances, been thoughtfully examined.
Regarding biological change per se, I see little reason to argue against the basic position developed by Wiley and Brooks: that organic change is a cumulative product of restrictions inherent in its own history. The point I wish to make, however, is that I believe their model focuses attention on a limited aspect of a more general process of evolution that cannot be neglected even when explanations for purely "biological" phenomena are sought. It is most fundamentally [[p. 271]] the spatial interaction structure that evolves on the earth's surface; individual populations are transient elements within that structure, contributing to it for periods of time varying on the basis not only of their own histories, but locational circumstances as well. Disequilibrium attending the early stages of this process must eventually be resolved when the biotic sector reaches the limit of its potential to further reduce the inefficiencies inherent in the operation of resource return pathways. As a spatial process, the dynamic equilibrium regime that follows will likely be characterized by increasing domination by highly flexible populations: where local environmental deterioration occurs, large-ranging, highly adaptable forms will simply be able to withdraw to more favorable locations, whereas smaller-ranging specialists will be more likely to go extinct.
8. FINAL COMMENTS
I will anticipate an obvious criticism of the entire chain of thought presented here by stating flatly that I see no way of directly testing the idea that the potential energy feedback loop in Figure 2 depicting the role of spatial interaction actually exists. This represents a comment on what I view as proper methodology, however, and not an apology. Purely biological tests (i.e., involving analysis of the biology of organisms or populations) are out of the question, because the adaptations that must at one level or another be the focus of biological inquiry contain almost no information relevant to the sum process envisioned (it is worthwhile in this context to recall the warning of Maruyama [77] regarding the impracticability of attempts to build normative models from historical descriptions of process). Nonetheless, it seems that indirect tests of the hypothesis should be possible. Given the hypothesis that spatial order devolves as a function of ordered processes, we might be able to demonstrate through normative studies of distributional ranges that the spatial distribution of stress levels supposedly responsible for ordering the biotic sector into spatial patterns in fact [[p. 272]] does so. Success in such attempts will support the contention that changes in available potential energy are being produced, and accepting this will pave the way for any number of more biologically-oriented analyses involving the spatial arrangement of biological attributes related thereto, and their historical development (e.g., the evolution of clines). The latter cannot be investigated first, because we have already allowed ourselves that change in distributional range mirrors evolutionary change in general, and to abandon this position before demonstrating that distributional patterns can in fact be interpreted as having resulted from the process posited is to regress toward the adaptation (process) yields adaptation (structure) circularity.
In a study described elsewhere [104] an operational model of the preceding set of deductions regarding the dynamics of distribution was constructed, and a number of specific tests on actual distribution patterns were carried out. Most of the findings were consistent with predictions initially deduced. For this kind of model, however, there can be no single validating test. A more extensive examination of actual distribution patterns is now in planning in the hope of providing a body of empiricism that can be subjected to discussion. Further development of the ideas presented here will have to wait until the completion of that work.
NOTE
1. As an aside, it should be noted that we cannot accept here the reasoning that the mere existence of such a resource makes it a selection factor ipso facto. While true its utilization would represent a necessary condition for existence, this does not argue that it must represent a necessary condition for adaptive change. Countering the latter claim is not difficult: all we need recognize is a variation-generating process which is in equilibrium with the "selecting" capabilities of the resource. In this view, the variation removed as a direct result of inability to process the resource leaves a gene pool capable of reproducing it in the next generation. (This understanding, I submit, is more consistent with the still useful Darwinian tenet that natural selection will produce organisms no better adapted than they need to be.) We must [[p. 273]] look elsewhere, I believe, for an understanding of the relation between such "ubiquitous" resources and the overall progression of organic change. In a work in preparation, I argue that this kind of resource, while incapable of acting as a direct selection agent, may yet play an important role in evolution by forcing selective extinction (especially of overspecialized forms).
REFERENCES
1. Andrewartha, H.G., and Birch, L.C. (1954). The distribution and abundance of animals. -- Chicago: Univ. of Chicago Press.
2. Ashby, W.R. (1956). An introduction to cybernetics. -- London: Chapman and Hall.
3. Baker, H.G., and Stebbins, G.L., eds. (1965). The genetics of colonizing species. -- New York and London: Academic Press.
4. Baker, R.G.V. (1982). Place utility fields. -- Geogr. Anal. 14: 10-28.
5. Barrett, G.W., and Rosenberg, R., eds. (1981). Stress effects on natural ecosystems. -- New York: John Wiley and Sons.
6. Bartholomew, G.A. (1958). The role of physiology in the distribution of terrestrial vertebrates. In C.L. Hubbs, ed., Zoogeography, 81-95. -- Washington, D.C.: Publication No. 51, American Association for the Advancement of Science.
7. Bernier, R. (1984). The species as an individual: facing essentialism. -- Syst. Zool. 33: 460-469.
8. Bertalanffy, L. von (1968). General systems theory. -- New York: George Braziller, Inc.
9. Bookstein, F. (1983). Comment on a "nonequilibrium" approach to evolution. -- Syst. Zool. 32: 291-300.
10. Boulding, K. (1956). General systems theory: the skeleton of science. -- General Systems Yearbook 1: 11-17.
11. Brookfield, J.F.Y. (1982). Adaptation and functional explanation in biology. -- Evol. Theor. 5: 281; 290.
12. Brooks, D.R., and Wiley, E.O. (1985). Nonequilibrium thermodynamics and evolution: responses to Bookstein and Wicken. -- Syst. Zool. 34: 89-97.
13. Brown, J.H., and Gibson, A.C. (1983). Biogeography. -- St. Louis: C.V. Mosby Co.
14. Brown, L.A. (1981). Innovation diffusion. -- London: Methuen.
15. Buffon, G.-L.L., Comte de (1749-1803). Histoire naturelle, generale et particuliere, avec la description du cabinet du roy. 44 vols. -- Paris: Imprimerie Royale.
16. Cain, S.A. (1947). Characteristics of natural areas and factors in their development. -- Ecol. Monogr. 17: 185-200.
17. Carson, M.A. (1969). Models of hillslope development under mass failure. -- Geogr. Anal. 1: 76-100.
18. Chorley, R.J., and Kennedy, B.A. (1971). Physical geography; a systems approach. -- London: Prentice-Hall.
[[p. 274]] 19. Cloud, P. (1976). Beginnings of biospheric evolution and their biogeochemical consequences. -- Paleobiology 2: 351-387.
20. Cloudsley-Thompson, J.L., and Chadwick, M.J. (1964). Life in deserts. -- Philadelphia: Dufour Editions.
21. Conrad, M. (1983). Adaptability. -- New York: Plenum Press.
22. Cope, E.D. (1887). The origin of the fittest: essays on evolution. -- New York: D. Appleton & Co.
23. Darwin, C. (1871). The descent of man and selection in relation to sex. -- London: John Murray.
24. Davidson, J., and Andrewartha, H.G. (1948). Annual trends in a natural population of Thrips imaginis (Thysanoptera) -- J. Anim. Ecol. 17: 193-222.
25. Dury, G.H. (1981). An introduction to environmental systems. -- London and Exeter, N.H.: Heinemann Educational Books.
26. Eldredge, N. (1981). Discussion of 'The riddle of dispersal: dispersal theories and how they affect vicariance biogeography', by M.D.F. Udvardy. In G. Nelson and D.E. Rosen, eds., Vicariance biogeography; a critique, 34-38. -- New York: Columbia Univ. Press.
27. Eldredge, N., and Gould, S.J. (1972). Punctuated equilibria: an alternative to phyletic gradualism. In T.J.M. Schopf, ed., Models in paleobiology, 82-115. -- New York: Freeman, Cooper, and Co.
28. Elton, C.S. (1958). The ecology of invasions by animals and plants. -- New York: Wiley.
29. Franz, E.H. (1981). A general formulation of stress phenomena in ecological systems. In G.W. Barrett and R. Rosenberg, eds., Stress effects on natural ecosystems, 49-54. -- Chichester, England: John Wiley and Sons.
30. Gates, D.M. (1962). Energy exchange in the biosphere. -- New York: Harper and Row.
31. Gates, D.M. (1970). Animal climates (where animals must live). -- Environ. Res. 3: 132-144.
32. Gates, D.M. (1980). Biophysical ecology. -- New York: Springer-Verlag.
33. Geiger, R. (1965). The climate near the ground (4th ed.). -- Cambridge, Mass.: Harvard Univ. Press.
34. Getis, A., and Boots, B. (1978). Models of spatial processes. -- Cambridge, England: Cambridge Univ. Press.
35. Ghiselin, M.T. (1966). On semantic pitfalls of biological adaptation. -- Philos. Sci. 33: 147-153.
36. Ghiselin, M.T. (1984). "Definition", "character" and other equivocal terms. -- Syst. Zool. 33: 104-110.
37. Gingerich, P.D. (1984). Punctuated equilibria -- where is the evidence? -- Syst. Zool. 33: 335-338.
38. Gleason, H.A. (1926). The individualistic concept of the plant association. -- Bull. Torrey Bot. Club 53: 7-26.
39. Gleason, H.A. (1939). The individualistic concept of the plant community. -- Am. Midl. Nat. 21: 92-110.
40. Gordon, M.S., Bartholomew, G.A., Grinnell, A.D., Jorgensen, C.B., and White, F.N. (1981). Animal physiology: principles and adaptations (4th ed.). -- New York: Macmillan.
41. Goudge, T.A. (1961). The ascent of life. -- Toronto: Univ. of Toronto Press.
[[p. 275]] 42. Gould, S.J., and Eldredge, N. (1977). Punctuated equilibria: the tempo and the mode of evolution reconsidered. -- Paleobiology 3: 115-151.
43. Gould, S.J., and Lewontin, R.C. (1979). The spandrels of San Marco and the Panglossian Paradigm: a critique of the adaptationist programme. -- Proc. R. Soc. London, Ser. b: 581-598.
44. Gould, S.J., and Vrba, E.S. (1982). Exaptation -- a missing term in the science of form. -- Paleobiology 8: 4-15.
45. Grant, P.R. (1978). Dispersal in relation to carrying capacity. -- Proc. Nat. Acad. Sci. 75: 2854-2858.
46. Greer-Wooten, B. (1972). The role of General Systems Theory in geographical research. -- Discussion Paper 3, Dept. of Geography, York University.
47. Grene, M. (1971). Two evolutionary theories. In R. Munson, ed., Man and nature, 139-167. -- New York: Delta.
48. Griffith, D.A., ed. (1983). Evolving geographical structures. -- Hingham, Mass.: Kluwer Boston.
49. Hadley, N.F., ed. (1975). Environmental physiology of desert organisms. -- Stroudsburg, Pa.: Dowden, Hutchinson and Ross.
50. Hagerstrand, T. (1967). Innovation diffusion as a spatial process. -- Chicago: Univ. of Chicago Press.
51. Harvey, D. (1967). Models of the evolution of spatial patterns in human geography. In R.J. Chorley and P. Haggett, eds., Models in geography. -- London: Methuen.
52. Holling, C.S. (1969). Stability in ecological and social systems. In G.M. Woodwell and H.H. Smith, eds., Diversity and stability in ecological systems, 128-141. -- Upton, New York: Brookhaven National Laboratory Publication No. 22.
53. Holling, C.S. (1973). Resilience and stability of ecological systems. -- Annu. Rev. Ecol. Syst. 4: 1-24.
54. Huggett, R. (1980). Systems analysis in geography. -- Oxford: Clarendon Press.
55. Huntington, E. (1924). Civilization and climate. 3rd ed. -- New Haven: Yale Univ. Press.
56. Hutchinson, G.E. (1964). The influence of the environment. -- Proc. Nat. Acad. Sci. 51: 930-934.
57. Hutchinson, G.E. (1978). An introduction to population ecology. -- New Haven: Yale Univ. Press.
58. Kimura, M. (1968). Evolutionary rate at the molecular level. -- Nature 217: 624-626.
59. Kimura, M. (1983). The neutral theory of molecular evolution. In M. Nei and R.K. Koehn, eds., Evolution of genes and proteins, 208-233. -- Cambridge, England: Cambridge Univ. Press.
60. Kimura, M. (1983). The neutral theory of molecular evolution. -- Cambridge, England: Cambridge Univ. Press.
61. King, J.L., and Jukes, T.H. (1969). Non-Darwinian evolution: random fixation of selectively neutral mutations. -- Science 164: 788-798.
62. Klopfer, R.P., and MacArthur, R.H. (1960). Niche size and faunal diversity. -- Am. Nat. 94: 293-300.
63. Kuppers, B.-O. (1983). Molecular theory of evolution. -- Berlin: Springer-Verlag.
[[p. 276]] 64. Lack, D. (1954). The natural selection of animal numbers. -- New York: Oxford Univ. Press.
65. Lack, D. (1971). Ecological isolation in birds. -- Oxford and Edinburgh: Blackwell Scientific Publications.
66. Lamarck, J.B.P.A. de M. (1809). Philosophie zoologique. 2 vols. -- Paris: Dentu.
67. Lenz, R.D. (1977). Applications of information theory in point pattern analysis. -- PhD. Dissertation, Dept. of Geography, Rutgers Univ., New Brunswick, New Jersey.
68. Levins, R. (1968). Evolution in changing environments. -- Princeton, New Jersey: Princeton Univ. Press.
69. Lewontin, R.C. (1969). The meaning of stability. In G.M. Woodwell and H.H. Smith, eds., Diversity and stability in ecological systems, 13-24. -- Upton, New York: Brookhaven National Laboratory Publication No. 22.
70. Lewontin, R.C. (1984). Adaptation. In E. Sober, ed., Conceptual issues in evolutionary biology, 235-251. -- Cambridge, Mass.: MIT Press.
71. Lovtrup, S. (1983). Victims of ambition: comments on the Wiley and Brooks approach to evolution. -- Syst. Zool. 32: 90-96.
72. MacArthur, R.H. (1972). Geographical ecology. -- New York: Harper and Row.
73. MacArthur, R.H., and Wilson, E.O. (1967). The theory of island biogeography. -- Princeton, New Jersey: Princeton Univ. Press.
74. Mares, M.A. (1980). Convergent evolution among desert rodents: a global perspective. -- Bull. Carnegie Mus. 16.
75. Margalef, R. (1969). Diversity and stability: a practical proposal and model of interdependence. In G.M. Woodwell and H.H. Smith, eds., Diversity and stability in ecological systems, 25-37. -- Upton, New York: Brookhaven National Laboratory Publication No. 22.
76. Maruyama, M. (1960). Morphogenesis and morphostasis. -- Methodos 12: 251-296.
77. Maruyama, M. (1963). The second cybernetics: deviation-amplifying mutual causal processes. -- Am. Scien. 51: 164-179.
78. May, R.M. (1973). Stability and complexity in model ecosystems. -- Princeton, New Jersey: Princeton Univ. Press.
79. McIntosh, R.P. (1967). The continuum concept of vegetation. -- Bot. Rev. 33: 130-187.
80. Meentemeyer, V., and Elton, W. (1977). The potential implementation of biogeochemical cycles in biogeography. -- Prof. Geogr. 29: 266-271.
81. Mooney, H.A., ed. (1977). Convergent evolution in Chile and California. -- Stroudsburg, Pa.: Dowden, Hutchinson and Ross.
82. Nicholson, A.J. (1957). The self-adjustment of populations to change. -- Cold Spring Harbor Symp. Quant. Biol. 22: 153-173.
83. Odum, E.P. (1971). Fundamentals of ecology (3rd ed.). -- Philadelphia: W.B. Saunders Co.
84. Odum, H.T. (1967). Work circuits and system stress. In Primary productivity and mineral cycling in natural ecosystems; a symposium, 81-138. -- Ecol. Soc. of America.
[[p. 277]] 85. Odum, H.T. (1971). Environment, power, and society. -- New York: Wiley-Interscience.
86. Olsson, G. (1970). Explanation, prediction, and meaning of variance: an assessment of distance interaction models. -- Econ. Geogr. 46: 223-233.
87. Oosting, H.J. (1956). The study of plant communities (2nd ed.). -- San Francisco: W.H. Freeman and Co.
88. Orians, G.H., and Solbrig, O.T., eds. (1977). Convergent evolution in warm deserts. -- Stroudsburg, Pa.: Dowden, Hutchinson and Ross.
89. Parsons, P.A. (1983). The evolutionary biology of colonizing species. -- Cambridge, England: Cambridge Univ. Press.
90. Penny, D. (1983). Charles Darwin, gradualism and punctuated equilibria. -- Syst. Zool. 32: 72-74.
91. Penny, D. (1985). Two hypotheses on Darwin's gradualism. -- Syst. Zool. 34: 201-205.
92. Pianka, E.R. (1970). On r and K selection. -- Am. Nat. 104: 592-597.
93. Pielou, E.C. (1974). Population and community ecology. -- New York: Gordon and Breach Science Publishers.
94. Ricklefs, R.E., and Cox, G.W. (1972). Taxon cycles in the West Indian avifauna. -- Am. Nat. 106: 195-219.
95. Ruse, M. (1973). The philosophy of biology. -- London: Hutchinson and Co.
96. Saarinen, E., ed. (1982). Conceptual issues in ecology. -- Dordrecht: D. Reidel Publishing Co.
97. Scheltema, R.S. (1973). Dispersal of the protozoan Folliculina simplex dons (Ciliophora, Heterotricha) throughout the North Atlantic Ocean on the shells of gastropod veliger larvae. -- J. Marine Res. 31: 11-20.
98. Schmidt-Neilsen, K. (1964). Desert animals; physiological problems of heat and water. -- Oxford, England: Oxford Univ. Press.
99. Schrodinger, E. (1945). What is life? -- New York: Macmillan.
100. Shannon, C.E., and Weaver, W. (1949). The mathematical theory of communication. -- Urbana, Illinois: Univ. of Illinois Press.
101. Sheppard, E.S. (1979). Geographic potentials. -- Ann. Assoc. Am. Geogr. 69: 438-447.
102. Simpson, G.G. (1953). Evolution and geography. -- Eugene, Oregon: Oregon State System of Higher Education.
103. Simpson, G.G. (1965). The geography of evolution. -- Philadelphia and New York: Chilton.
104. Smith, C.H. (1984). The dynamics of animal distribution: an evolutionary / ecological model. -- Phd. Dissertation, Dept. of Geography, Univ. of Illinois, Champaign-Urbana, Illinois.
105. Smith, C.H. (1985). A general approach to the study of spatial systems. I. The relational representation of measureable attributes. -- Intern. J. Gen. Systems (in press).
106. Smith, C.H. (1985). A general approach to the study of spatial systems. II. Two examples of application. -- Intern. J. Gen. Systems (in press).
[[p. 278]] 107. Smith, F.E. (1961). Density dependence in the Australian thrips. -- Ecology 42: 403-407.
108. Stanley, S.M. (1979). Macroevolution; pattern and process. -- San Francisco: W.H. Freeman.
109. Thiery, R.G. (1982). Environmental instability and community diversity. -- Biol. Revs. 57: 691-710.
110. Trudinger, P.A., Swaine, D.J., and Skyring, G.W. (1979). Biogeochemical cycling of elements -- general considerations. In P.A. Trudinger and D.J. Swaine, eds., Biogeochemical cycling of mineral-forming elements, 1-27. -- Amsterdam: Elsevier.
111. Van Valen, L. (1971). Group selection and the evolution of dispersal. -- Evolution 25: 591-598.
112. Van Valen, L. (1971). The history and stability of atmospheric oxygen. -- Science 171: 439-443.
113. Van Valen, L. (1973). A new evolutionary law. -- Evol. Theor. 1: 1-33.
114. Waesberghe, H. van (1982). Towards an alternative evolution model. -- Acta Biotheoret. 31: 3-28.
115. Wallace, A.R. (1869). Sir Charles Lyell on geological climates and the origin of species. -- Quart. Rev. 126: 359-394.
116. Weiss, P.A. (1971). The basic concept of hierarchic systems. In P.A. Weiss, ed., Hierarchically organized systems in theory and practice, 1-43. -- New York: Hafner.
117. Whittaker, R.H. (1953). A consideration of the climax theory: the climax as a population and pattern. -- Ecol. Monogr. 23: 41-78.
118. Whittaker, R.H. (1962). Classification of natural communities. -- Bot. Rev. 28: 1-239.
119. Whittaker, R.H. (1973). Approaches to classifying vegetation. In R.H. Whittaker, ed., Ordination and classification of communities, 323-354. -- The Hague: Dr. W. Junk, Publishers.
120. Wicken, J.S. (1983). Entropy, information, and nonequilibrium evolution. -- Syst. Zool. 32: 438-443.
121. Wiebes, J.T. (1982). L'adaptation evolutive. -- Acta Biotheoret. 31: 239-243.
122. Wiley, E.O., and Brooks, D.R. (1982). Victims of history -- a nonequilibrium approach to evolution. -- Syst. Zool. 31: 1-24.
123. Wiley, E.O., and Brooks, D.R. (1983). Nonequilibrium thermodynamics and evolution: a response to Lovtrup. -- Syst. Zool. 32: 209-219.
124. Wilson, A.G. (1970). Entropy in urban and regional planning. -- London: Pion.
125. Wilson, D.S. (1980). The natural selection of populations and communities. -- Menlo Park, Calif.: Benjamin / Cummings Publ. Co.
126. Wilson, E.O. (1961). The nature of the taxon cycle in the Melanesian ant fauna. -- Am. Nat. 95: 169-193.
127. Windley, B.F., ed. (1975). The early history of the earth. -- New York: Wiley-Interscience.
128. Zadeh, L.A. (1969). The concepts of system, aggregate and state in system theory. In L.A. Zadeh and E. Polak, eds., System theory, 3-42. -- New York: McGraw-Hill.
*
*
*
*
*
Copyright 1986, 2002 by Charles H. Smith. All rights reserved.