|
Because of the ease of making spectrum-to-structure relations, IR and Raman spectroscopies are excellent methods for analyzing atmospheric reactions. Thus, atmospheric research groups have made IR observations in the laboratory of these important atmospheric components. These measurements have contributed to the overall understanding of the sources, sinks and chemical reactions of each molecule in the upper and lower atmospheres. It is in the first few nanoseconds and microseconds after photoinitiation, before the system is able to reach kinetic equilibrium, that such reaction sequences branch into a tangled web of interconnected species. We study kinetics as they transpire using time-resolved IR and Raman spectroscopy. By initiating a reaction with a temporally-short radiation source (the “pump”) and then “probing” the vibrational spectrum at different times following the pump, we will monitor the spectrum as it evolves in time, revealing the underlying kinetics. One time-resolved approach which has gained particular popularity is time-resolved Raman spectroscopy (see below).
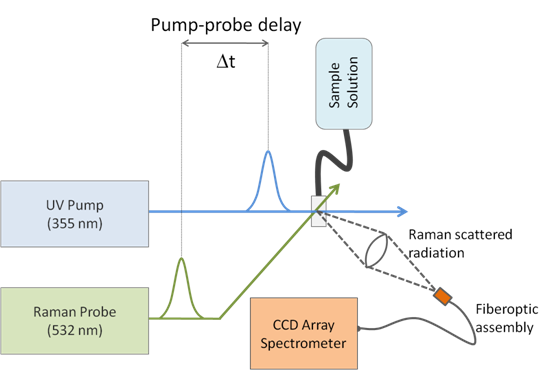
In normal Raman spectroscopy, frequency multiplexing (measuring all frequencies all the time) is accomplished by collecting the inelastically scattered radiation from a sample. As shown in the figure above, this is accomplished by sending a pulsed laser pulse (15 ns at 532 nm) into our sample, and dispersing the scattered radiation using charged-couple device array detector. To obtain good temporal resolution, the measurement process must be faster than the reaction times involved. A pump beam (also 15 ns, but at 355 nm, for example) can be used to excite molecules prior to measuring their spectrum. The changes to the raman spectrum as a function of Dt reveal the identity of products and intermediates in a set of branched reactions as they unfold. Taking a slice of the spectra through time at a given frequency, one may plot the intensity of a given peak as a function of this pump-probe time to acquire the concentrations of each product and reactant as a function of Dt. By the Beer-Lambert Law, the changes in absorption spectrum as a function of time are directly related to the concentrations of the different components being studied, mapping spectroscopy directly to kinetic data. In our lab, kinetic models will be compared by fitting rate constants and branching ratios to the observed data, allowing us to critically compare proposed reaction mechanisms.
|
|
Time-resolved vibrational spectroscopy |